REDWOOD CITY, Calif. and SHANGHAI, China, Oct. 27, 2023 (GLOBE NEWSWIRE) -- Coherus BioSciences, Inc. (“Coherus”, NASDAQ: CHRS), and Shanghai Junshi Biosciences Co., Ltd. (Junshi Biosciences, HKEX: 1877; SSE: 688180) today announced that the U.S. Food and Drug Administration (FDA) approved Loqtorzi™ (toripalimab-tpzi) in combination with cisplatin and gemcitabine for the first-line treatment of adults with metastatic or recurrent locally advanced NPC, and as monotherapy for the treatment of adults with recurrent, unresectable, or metastatic NPC with disease progression on or after platinum-containing chemotherapy. The approval was based on results of the JUPITER-02 Phase 3 study and the POLARIS-02 Phase 2 study and is irrespective of a patient’s PD-L1 status. Loqtorzi is a next-generation, programmed death receptor-1 (PD-1) monoclonal antibody that blocks PD-1 ligands PD-L1 and PD-L2 with high potency at a unique site on the PD-1 receptor, enabling the immune system to activate and kill the tumor.
In the JUPITER-02 Phase 3 study, Loqtorzi combined with chemotherapy significantly improved progression-free survival (PFS), reducing the risk of disease progression or death by 48% compared to chemotherapy alone. Loqtorzi also demonstrated a statistically significant and clinically meaningful improvement in overall survival (OS), with treatment resulting in a 37% reduction in the risk of death versus chemotherapy alone.
The safety profile of Loqtorzi was consistent with the PD-1 inhibitor class. The incidence of Grade ≥3 adverse events (AEs) (89.7% vs 90.2%) and fatal AEs (3.4% vs 2.8%) was similar between the two arms. AEs leading to discontinuation of Loqtorzi versus placebo (11.6% vs 4.9%), immune-related adverse events (irAEs) (54.1% vs. 21.7%), and Grade ≥3 irAEs (9.6% vs. 1.4%) were more frequent in the Loqtorzi arm.
In the POLARIS-02 clinical study Loqtorzi demonstrated durable antitumor activity in patients with recurrent or metastatic NPC who failed previous chemotherapy, with an objective response rate (ORR) of 20.5%, a disease control rate (DCR) of 40.0%, and a median OS of 17.4 months with an acceptable safety profile.
NPC is an aggressive cancer that starts in the nasopharynx, the upper part of the throat behind the nose and near the base of skull. Due to the location of the primary tumor, surgery is rarely an option, and patients with localized disease are treated primarily with radiation and chemotherapy. Loqtorzi is the first FDA-approved agent for NPC patients.
“Loqtorzi’s first approval is a pivotal event for Coherus as an innovative oncology company. As a next generation PD-1 inhibitor it is the keystone of our I-O strategy to extend cancer patient survival as shown with the impressive results in NPC,” said Denny Lanfear, Chairman and Chief Executive Officer of Coherus. “We are particularly excited to now turn our attention to developing Loqtorzi across multiple tumor types in combination with I-O agents that target the tumor microenvironment, such as our IL27-targeted antibody, casdozokitug, and our CCR8 inhibitor CHS-114, potentially greatly expanding the number of cancer patients achieving improved survival benefit.”
“Today’s FDA approval of Loqtorzi is very encouraging for those living with NPC who currently have very limited treatment options and are in need of new therapies to treat this aggressive and life-threatening form of cancer,” said Jong Chul Park, M.D., Assistant Professor, Harvard Medical School and attending physician at the Center for Head and Neck Cancers at Massachusetts General Hospital Cancer Center. “Loqtorzi is a new treatment option that has demonstrated the ability to significantly improve PFS and OS and should quickly emerge as the new standard of care when used in combination with chemotherapy.”
The recommended Loqtorzi dose with cisplatin and gemcitabine is 240 mg every three weeks until disease progression, unacceptable toxicity, or up to 24 months. The recommended Loqtorzi dose as a single agent for previously treated NPC is 3 mg/kg every two weeks until disease progression or unacceptable toxicity.
Loqtorzi is expected to be available in the United States in Q1 2024.
“The impressive results from JUPITER-02 and POLARIS-02 have provided conclusive evidence that establishes toripalimab, in combination with chemotherapy or as monotherapy, as the standard therapy for advanced NPC,” said Professor Ruihua Xu of Sun Yat-sen University Cancer Center, the principal investigator of JUPITER-02 and POLARIS-02. “This great achievement was only made possible through the solid foundation laid by countless oncology experts over decades of in-depth research, as well as the selfless dedication of the patients and research teams involved in our toripalimab studies. We hope that this promising therapy will close the treatment gap for international NPC patients struggling to find effective therapies, bringing them renewed hope for better survival.”
“We’re excited to reach another significant company milestone of ‘going overseas’,” said Dr. Ning LI, Chief Executive Officer of Junshi Biosciences. “Following etesevimab, toripalimab has become Junshi Biosciences’ second product to receive FDA approval for commercialization—an achievement that will further enhance the company’s international presence. Currently, the establishment of toripalimab’s global commercialization network is in progress, and the network aims to span over 50 countries. In accordance with the company’s ‘In China, For Global’ strategy, we will continue working with our collaborators to promote the commercialization of toripalimab in other regions, in order to provide innovative and high-quality drugs from China to more patients overseas.”
Source: https://www.drugs.com/newdrugs/fda-approves-loqtorzi-toripalimab-tpzi-all-lines-recurrent-metastatic-nasopharyngeal-carcinoma-npc-6130.html
The FAI climbed 5.9 percent year-on-year in the first 11 months of 2018, quickening from the 5.7-percent growth in Jan-Oct, the National Bureau of Statistics (NBS) said Friday in an online statement.
The key indicator of investment, dubbed a major growth driver, hit the bottom in August and has since started to rebound steadily.
In the face of emerging economic challenges home and abroad, China has stepped up efforts to stabilize investment, in particular rolling out measures to motivate private investors and channel funds into infrastructure.
Friday's data showed private investment, accounting for more than 60 percent of the total FAI, expanded by a brisk 8.7 percent.
NBS spokesperson Mao Shengyong said funds into weak economic links registered rapid increases as investment in environmental protection and agriculture jumped 42 percent and 12.5 percent respectively, much faster than the average.
In breakdown, investment in high-tech and equipment manufacturing remained vigorous with 16.1-percent and 11.6-percent increases respectively in the first 11 months. Infrastructure investment gained 3.7 percent, staying flat. Investment in property development rose 9.7 percent, also unchanged.
 English
English

















































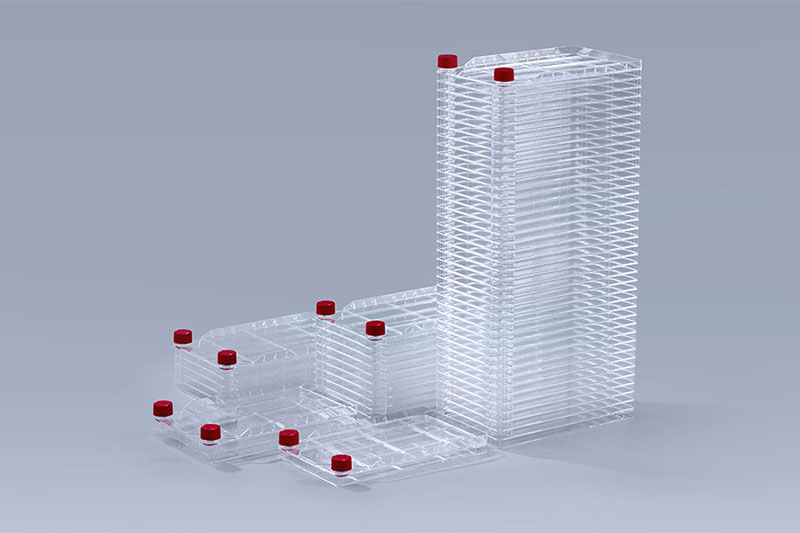 Cell Factory
Cell Factory Cell Culture Erlenmeyer Flask
Cell Culture Erlenmeyer Flask PETG Media Bottles
PETG Media Bottles